The idea of correlatin between the molecular geometry and the number of valence electrons (both shared and unshared) was originally proposed in 1939 by Ryutaro Tsuchida in Japan, and was independently presented in a Bakerain Lecturein 1940 Nevil Sidgwick and Herbert Powell of the University of oxford In 1957, Ronald Gillespie and Ronald Sydney Nyhlom of University college of London refined this concept into a more detailed theory, capable of choosing between various alternative geometries.
Rule;
- IF the central atom of the molecule is surrounded by only bonding electron pair and not by non bonding electron pair, the geometry of the molecule will be regular as predicted by hybridization.
- When the central atom in a molecule is surrounded by both bond and lone pair, the molecule doesnt have regular shape.
- Presence of lone pair on the central atom cause slight distortion of the bond angle from the ideal shape. The extent of repulsion between different electron pair is in the order of; l.p-l.p repulsion> l.p-b.p repulsion>b.p-b.p repulsion
- The magnitude of a repulsion between bonding pairs of electrons depends on the electronegativity difference between the central atom and other atoms.
- Double bond cause more repulsion than single bond and triple bond cause more repulsion than double bond.
| Molecule Type | Shape[12] | Electron arrangement†[12] | Geometry‡[12] | Examples |
|---|---|---|---|---|
| AX2E0 | Linear | BeCl2,[1] HgCl2,[1] CO2[11] | ||
| AX2E1 | Bent |  |  | NO− 2,[1] SO2,[12] O3,[1] CCl2 |
| AX2E2 | Bent | 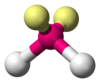 |  | H2O,[12] OF2[17] |
| AX2E3 | Linear |  | XeF2,[12] I− 3,[18] XeCl2 | |
| AX3E0 | Trigonal planar |  |  | BF3,[12] CO2− 3,[19] NO− 3,[1] SO3[11] |
| AX3E1 | Trigonal pyramidal |  |  | NH3,[12] PCl3[20] |
| AX3E2 | T-shaped |  |  | ClF3,[12] BrF3[21] |
| AX4E0 | Tetrahedral | 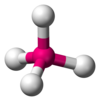 |  | CH4,[12] PO3− 4, SO2− 4,[11] ClO− 4,[1] XeO4[22] |
| AX4E1 | Seesaw (also called disphenoidal) |  | 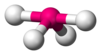 | SF4[12][23] |
| AX4E2 | Square planar |  |  | XeF4[12] |
| AX5E0 | Trigonal bipyramidal |  |  | PCl5[12] |
| AX5E1 | Square pyramidal | 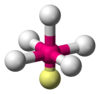 | 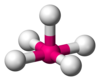 | ClF5,[21] BrF5,[12] XeOF4[11] |
| AX5E2 | Pentagonal planar |  |  | XeF− 5[14] |
| AX6E0 | Octahedral | 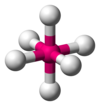 | 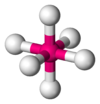 | SF6,[12] WCl6[24] |
| AX6E1 | Pentagonal pyramidal |  |  | XeOF− 5,[13] IOF2− 5[13] |
| AX7E0 | Pentagonal bipyramidal[11] | 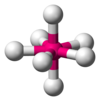 | 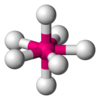 | IF7[11] |
| AX8E0 | Square antiprismatic[11] |  |  | IF− 8, ZrF4− 8, ReF− 8 |
| AX9E0 | Tricapped trigonal prismatic (as drawn) OR capped square antiprismatic | 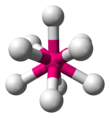 |  | ReH2− 9[15] |
- Reference:
- Dasu Paudel
- Wikipedia
- Advance Inorganic Chemistry
No comments:
Post a Comment